
Bed and Stretcher Product Training Modules Simplified, accessible, and flexible training. Hillrom’s on-line modular bed and stretcher product training, simplifies the training process by creating flexible segments that allow a user to select and review specific product features. Air-fluidized therapy beds were provided for patients who required vasopressors and mechanical ventilation for at least 24 hours postoperatively. While on therapy beds, only 1 of 27 patients developed a stage I ulcer versus 40 ulcers developed in 25 patients prior to therapy bed implementation despite already being a high-risk population for.
-
| 510(k) | | | DeNovo | | | Registration & Listing | | | Adverse Events | | | Recalls | | | PMA | | | HDE | | | Classification | | | Standards |
| CFR Title 21 | | | Radiation-Emitting Products | | | X-Ray Assembler | | | Medsun Reports | | | CLIA | | | TPLC |
|
| HILL-ROM, INC. CLINITRON BED BED, AIR FLUIDIZED | Back to Search Results |
| | Model Number 0800 | | Device Problems Loss of Power (1475); Device Displays Incorrect Message (2591) | | Patient Problem Injury (2348) | | Event Date 12/12/2014 | | Event Type Injury | | Event Description | Hill-rom received a report from the account stating the unit was alarming and shutting down. The pt developed stage 3 wounds and was admitted to the hosp. The bed was located at the account. There was a pt injury reported. This report was filed in our complaint handling system as complaint # (b)(4). | | Manufacturer Narrative | The hill-rom tech found the care givers placed an egg crate foam under the pt which left her on the hard surface, which would have contributed to further skin break down. The tech also found the bed had heater failures. Also had a hole in bottom bladder cover at head section causing bead leak and several other worn areas. Filter sheet badly stained as well. Further assessment found that the diffuser board was clogged, heavily soiled beads, and bad cable assembly temperature cutoff which caused the heater failure. The technician replaced the failed parts to resolve the issue. Development of pressure ulcers is multifactorial and cannot be only attributed to performance of the surface. Risk factors include protein-calorie malnutrition, microclimate (skin wetness caused by sweating or incontinence), diseases that reduce blood flow to the skin, such as arteriosclerosis, or diseases that reduce the sensation in the skin, such as paralysis or neuropathy. Position changes are key to pressure sore prevention and treatment. These changes need to be frequent, repositioning needs to avoid stress on the skin, and body positions need to minimize the risk of pressure on vulnerable areas. A search of the hill-rom maintenance records did not show hill-rom performed any preventive maintenance on this bed. It is unk if the facility performs preventive maintenance on its beds. | | Search Alerts/Recalls |
|
|
| New Search | Submit an Adverse Event Report |
| Type of Device | BED, AIR FLUIDIZED |
|---|
| Manufacturer (Section D) | | HILL-ROM, INC. | | batesville IN |
|
|---|
| Manufacturer Contact | | john cummings | | 1069 state route 46 east | | batesville, IN 47006 | | 8129312869 |
|
|---|
| MDR Report Key | 4417020 |
|---|
| MDR Text Key | 17579475 |
|---|
| Report Number | 1824206-2015-00058 |
|---|
| Device Sequence Number | 1 |
|---|
| Product Code | INX |
|---|
| Combination Product (Y/N) | N |
|---|
| Reporter Country Code | US |
|---|
| PMA/PMN Number | K964223 |
|---|
| Number of Events Reported | 1 |
|---|
| Summary Report (Y/N) | N |
|---|
| Report Source | Manufacturer |
|---|
| Source Type | User facility |
|---|
| Reporter Occupation |
|---|
| Type of Report | Initial |
|---|
| Report Date | 12/12/2014 |
|---|
| 1 Device Was Involved in the Event |
|---|
| 1 Patient Was Involved in the Event |
|---|
| Date FDA Received | 01/09/2015 |
|---|
| Is This An Adverse Event Report? | Yes |
|---|
| Is This A Product Problem Report? | No |
|---|
| Device Operator |
|---|
| Device MODEL Number | 0800 |
|---|
| Was Device Available For Evaluation? | Yes |
|---|
| Is The Reporter A Health Professional? | No |
|---|
| Date Manufacturer Received | 12/12/2014 |
|---|
| Was Device Evaluated By Manufacturer? | Yes |
|---|
| Is The Device Single Use? | No |
|---|
| Is this a Reprocessed and Reused Single-Use Device? | No |
|---|
| Type of Device Usage | Unkown |
|---|
| Patient TREATMENT DATA |
|---|
| Date Received: 01/09/2015 Patient Sequence Number: 1 |
|---|
|
|
One moment please...
Not A Member?Sign Up
Hillrom - Clinitron Rite Hite Air Fluidized Therapy Bed
by Hillrom
Clinitron Air Fluidized Bed Manual Transmission
DESCRIPTION

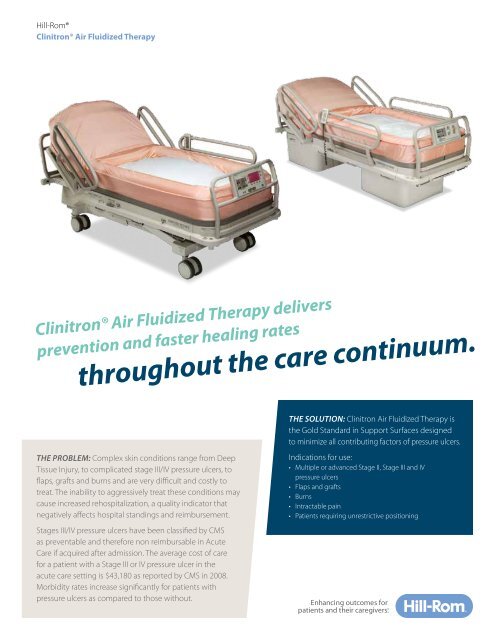
Clinitron Air Fluidized Therapy beds provide an ideal healing environment for compromised skin by minimizing the forces that cause tissue breakdown: pressure, shear, friction, heat and moisture. Clinitron Rite Hite system’s unique air fluidized therapy minimizes interface pressure, while maximizing the surface’s immersion and envelopment properties to support healing.
The Clinitron Air Fluidized Therapy bed utilizes advanced technology to provide the highest level of wound care for patients with complex, advanced wounds that are difficult to heal and expensive to manage.
FORUMSView All
Ask a New Question

Clinitron Air Fluidized Bed Manual Parts
SERVICE COMPANIESView All Bed Companies
Kci Clinitron Bed
FEATURES
- Clinitron Rite Hite Air Fluidized Therapy supports skin integrity by providing statistically lower interface pressure and superior microclimate management™ surfaces over traditional surfaces1
- Medical grade, silicone-coated bead fluidization promotes a flotation environment that can help improve patient comfort
- Hi/Lo adjustment can make patient positioning and egress easier and can provide a safer working height for caregivers.
- Electric Head of Bed articulation can help accommodate patient’s with respiratory complications
- Optional Clinitron Rite Hite system Patient Helper accessory can help patients change positions in bed and aid in transfers.
Additional Specifications
| Standard Length | 84' |
| Overall Length | 92.5' (231 cm) |
| Standard Width | 36' (Siderails off); 42' (Siderails on) |
| Standard Height | 21.5' |
| Therapeutic Patient Weight Limit | 350 lbs. |




